Diminishing returns in medical therapy
I’m a few months late, but I want to mention an editorial by Rodney Hayward that was published in The BMJ in December 2015. His topic is treatment of diabetes, but the principles he discusses also apply to other areas of medicine. The key concept is that even in high risk conditions such as diabetes, adding a second or third medication brings diminishing absolute returns as residual risk decreases as each additional treatment is added. He starts by describing the disturbing consequences of untreated or poorly managed diabetes, and how things have changed with modern therapies.
When I began my medical training in 1980, I commonly encountered patients whose bodies were ravaged by end stage complications of diabetes. These patients often had marked visual impairment, debilitating neuropathy, myopathies, and diabetes related renal insufficiency, well before age 65 years. I still occasionally see such individuals, but they are rare, and tend to come from the 10-15% of patients who still have poor glycemic control. Improvement in diabetes care is a medical success story, but increasing evidence suggests that overly aggressive treatment is an under-appreciated problem.
The problem is that focusing on relative treatment effects ignores the law of diminishing returns; past a certain point, additional reductions in HbA1c have limited benefit in absolute terms for most older patients with type 2 diabetes. Hayward explains:
Diminishing returns is a mathematical fact, not a theory. Try this simple experiment. Serially tear a piece of paper in half and throw one half away. You will notice that the relative effects never diminish (you reduce the piece of paper by half each time), but it doesn’t take long for the 50% you throw away to become tiny. The many patients with end stage diabetes we saw in the 1980s often spent years with poor control of both glycemia and blood pressure. They had no access to metformin, home blood glucose monitoring, angiotensin converting enzyme inhibitors, calcium channel blockers, and a host of other modern interventions. Each of these interventions substantially reduces disease progression and has an even larger effect on end stage diabetes complications. Because each intervention substantially reduces end stage complications, it should not be surprising that recent evidence has found intensive glycemic control to have a small absolute effect on end stage complications for most patients with type 2 diabetes. The law of diminishing returns predicts this result. Also, as the benefits of tighter glycemic control become smaller, the chances that treatment harms will outweigh treatment benefits become much greater.
Hayward ends by stating that the public good would best be served by focusing on the minority of diabetes patients who continue to be at substantial risk of diabetes-related morbidity and mortality and promoting more shared decision making with older diabetes patients who already have at least moderate blood glucose control.
The same principle of diminishing returns applies in other areas of medicine, such as in medications that reduce cardiovascular risk by lowering blood pressure or cholesterol. As the second and third medication is added, the patient’s risk of experiencing a cardiovascular event diminishes and in some cases a point can be reached where the absolute benefits become very small and it becomes difficult to tell whether benefits outweigh harms. When benefits become small, it can sometimes be hard to determine whether they exist at all or, perhaps, exist in only in patients with certain characteristics. See this post by Harlan Krumholz for a discussion of these issues in the area of treatment of high blood pressure.
In the area of cholesterol-lowering drugs, the new PCSK9 inhibitors have been in the news and I’ve previously discussed them on this blog (here, here and here). Two of these drugs, evolocumab and alirocumab, are approved in the U.S. and so far aren’t selling well. There are several reasons for that, including that the outcomes trials haven’t been completed yet and that the drugs are much more expensive than statins, almost all of which are available as generics. Another reason, related to the first two, is that insurance companies have imposed strict preauthorization requirements for these drugs. Another reason relates to the theme of this post, namely the diminishing returns from adding additional drugs. I’m going to take the treatment of heterozygous familial hypercholesterolemia (HeFH) as an example, specifically patients with HeFH who do not have clinical atherosclerotic cardiovascular disease and who are thus being treated to prevent a first event (i.e., “primary prevention”).
HeFH greatly increases the risk of developing premature atherosclerotic cardiovascular disease compared to individuals with normal levels of cholesterol. Before statins became available, the drugs that were available were not very effective. However, in recent decades, first moderate intensity and then high intensity statins were instituted as standard treatment of HeFH, often with additional drugs such as ezetimibe. According to UpToDate, atorvastatin can reduce LDL by up to 54% and rosuvastatin can reduce LDL by up to 63%. Ezetimibe can lower LDL by another 15% or so in patients on a statin. Given that most patients with HeFH have LDL in the 200s or below, a reduction of 50-60% achieves very reasonable LDL levels. There is evidence that even moderate doses of statins greatly reduce the risk of heart disease in HeFH patients who are being treated for primary prevention. A study published in JAMA in 2014 showed that young adults with HeFH have near-normal levels of atherosclerosis 10 years after initiation of statin therapy. The use of high intensity statins has been shown to greatly reduce the progression of atherosclerosis in adult HeFH patients (see here and here) even when compared to moderate statin therapy. Thus, HeFH patients who start treatment early and are able to reduce their LDL to normal or near-normal levels over many years with a statin or statin + ezetimibe often do not need an additional drug, as their risk is greatly reduced.
So which HeFH patients do need an additional LDL-lowering therapy, such as a PCSK9 inhibitor? To my knowledge, there are no risk calculators available to guide decisions in this area. HeFH patients who start with very high LDL, who can’t tolerate high doses of statins or can’t tolerate statins at all, who started treatment late, who have additional cardiovascular risk factors, or who have had imaging that shows significant subclinical atherosclerosis, are going to be at higher risk, on average. There is quite a bit of uncertainty involved, as with estimation of cardiovascular risk in general. In addition, there are personal preferences involved, as people vary greatly in terms of how much risk they are willing to live with.
Interestingly, a task force of the International Atherosclerosis Society just published a consensus statement in The Lancet Diabetes & Endocrinology that discusses some of the factors involved in determining cardiovascular risk in FH. Although the criteria they propose for use of additional therapies are more stringent than I foresee being adopted in the U.S., the paper contains some very useful discussion of the heterogeneity of cardiovascular risk in FH and ways of trying to predict who is at higher risk. I’m pasting in their proposed criteria below, in case anyone is interested, but I do recommend the entire paper.
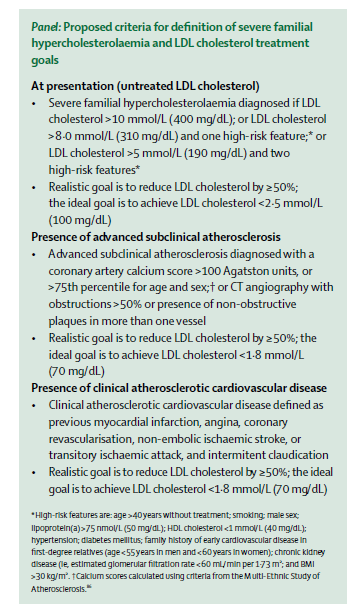
Posted on June 3, 2016, in cardiology, Uncategorized and tagged alirocumab, cardiovascular risk, cholesterol, diabetes, evolocumab, ezetimibe, familial hypercholesterolemia, Harlan Krumholz, hypertension, PCSK9 inhibitors, Rodney Hayward, statins. Bookmark the permalink. Leave a comment.
Leave a comment
Comments 0